[Chinese instrument network instrument research and development] Recently, the Li Yue Group of Institute of Micronanotechnology and Devices of the Institute of Solid State Physics, Chinese Academy of Sciences, Hefei University of Science and Technology, Professor Deng Zhaoxiang and Li Liangbin of the University of Science and Technology of China, cooperating with Professor Li Cuncheng of Jinan University. Advances have been made in the assembly of gold nanosphere dimers and new colorimetric sensing methods for plasmon coupling dynamics control. Related results were published on Advanced Functional Materials.
Colorimetric sensor device based on Au dimer/chitosan hydrogel
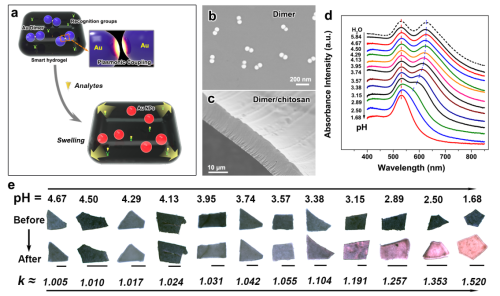
Colorimetric sensing is the use of changes in the optical properties of the sensing unit (absorption or emission) as an output signal to achieve the visual detection of the analyte. It has the characteristics of convenient carrying, simple operation, real-time monitoring in the field, and so on. In recent years, many colorimetric nanoparticle-based colorimetric sensor devices have been reported successively, and are widely used for the visual detection of heavy metal ions, food additives, toxic agents, etc. Therefore, the research of colorimetric sensors has attracted people's great interest. The principle of colorimetric sensing based on precious metal nanoparticles is to use the color change caused by the dispersion and polymerization phenomenon of noble metal nanoparticles triggered by the target analyte as an output signal, thereby realizing the visual detection of the analyte. However, this precious metal nanoparticle dispersion (or disassembly) and polymerization (or assembly) phenomenon, has a random controllability, resulting in low sensitivity of the sensor signal. In addition, the core unit (precious metal nanoparticles) is susceptible to agglomerates due to external interference and is not easily stored, resulting in poor stability. Therefore, it is still a challenge to develop colorimetric sensor devices with high sensitivity, high stability, and wide dynamic control range.
In response to this problem, researchers have successfully obtained large-scale, high-quality Au nanosphere dimers on the basis of successfully obtaining uniform-sized monodispersed Au nanospheres in the early stage, using silver ion-induced self-assembly and electrophoretic separation techniques, and presenting Au A new idea of ​​dimerization and intelligent hydrogels, using the high optical sensitivity and tunable gap-dependent properties of Au dimers, developed a new type of highly sensitive and highly stable Au-based dimer. Dynamically adjustable sensitivity range colorimetric sensor device. The sensor cleverly uses the characteristics that the hydrogel is rapidly expanded or shrunk due to stimulation by external substances, and successfully realizes the precise and dynamic control of the optical properties of the Au nanosphere dimer, thereby realizing a highly sensitive colorimetric sensor. The construction of the parts; In addition, the Au dimer embedded in the hydrogel film can effectively overcome its lack of spontaneous agglomeration and settlement, and has good stability. Further, the team rationally utilized the sensitivity of the hydrogel's degree of swelling inversely proportional to its degree of cross-linking to broaden the dynamic tunable range of the device's sensitivity, enabling sensitive detection of target analytes in different concentration ranges. This work provides a new approach for the study of dynamic precise control of plasmon coupling optical properties of Au nanosphere dimers, and provides new ideas for the construction of ultra-high sensitive and highly stable sensor parts.
The research work was supported by the national key R&D program, the National Natural Science Foundation of China, and the Natural Science Foundation of Anhui Province.
(Headline: Hefei Research Institute has made progress in the research of plasmonic coupling sensing based on the dynamic regulation of gold nanosphere dimerization)
Amine & Derivative
One, two, or three hydrogen atoms in an
ammonia molecule are substituted with an alkyl group to produce compounds
called first amines (primary amines), second amines (secondary amines), and
tertiary amines (tertiary amines), respectively. Their general formulas are:
RNH2 - primary amine, R2NH - secondary amine, R3N - tertiary amine. Amines are
widely present in the biological world and have extremely important
physiological effects. Therefore, the vast majority of drugs contain the
functional group of amines - amine groups. Proteins, nucleic acids, many
hormones, antibiotics, and alkaloids all contain amine groups that are complex
derivatives of amines. According to the different types of hydroxyl groups in the amine molecule connected to the nitrogen atom, amines can be divided into fatty amines and aromatic amines. If the amine molecule contains two or more amino groups (-NH2), it can be divided into dibasic amines and terbasamines according to the number of amino groups.
Amines are widely distributed in nature, most of which are produced by amino acid decarboxylation. For example, the industrial preparation of amines is mostly prepared by the reaction of ammonia with alcohol or haloalkanes, and the product is a mixture of amines at all levels, which is purified after fractionation. Catalytic reduction by aldehydes and ketones in the presence of ammonia can also obtain corresponding amines. In industry, amine compounds are often prepared by catalytic reduction of nitro compounds, nitriles, amides or nitrogen-containing heterocyclic compounds
Amines are used for a wide range of purposes. The earliest development of the dye industry was based on aniline. Some amines are necessary to maintain life activities, but some are also very harmful to life, many amine compounds have carcinogenic effects, especially aromatic amines, such as naphthylamine, benzidine and so on.
Amine Chemicals,Azodicarbonamide,Hydrazine Hydrate,Amine & Derivative
WUHAN RUISUNNY CHEMICAL CO. LTD , https://www.ruisunny-chem.com